



The safety and efficacy of MYCAPSSA were studied in 3 clinical trials.1-4
|
Study
|
Treatments studied | Number of participants | Outcome |
|
Phase 3
Open-Label Study
|
MYCAPSSA | N=155 |
|
|
OPTIMAL
|
MYCAPSSA vs placebo | N=56 |
|
|
MPOWERED
|
MYCAPSSA vs injectable SSA | N=146 |
|
| Study |
|
Phase 3
Open-Label Study
|
| Treatments studied |
| MYCAPSSA |
| Number of participants |
| N=155 |
| Outcome |
|
| Study |
|
OPTIMAL
|
| Treatments studied |
| MYCAPSSA vs placebo |
| Number of participants |
| N=56 |
| Outcome |
|
| Study |
|
MPOWERED
|
| Treatments studied |
| MYCAPSSA vs injectable SSA |
| Number of participants |
| N=146 |
| Outcome |
|







— Bonnie, MYCAPSSA patient
This is Bonnie's experience and may not be representative of every patient taking MYCAPSSA.








— Becky, MYCAPSSA patient
This is Becky's experience and may not be representative of every patient taking MYCAPSSA.
Most GI-related AEs are temporary. Encourage patients to stay in touch as you work through it together.



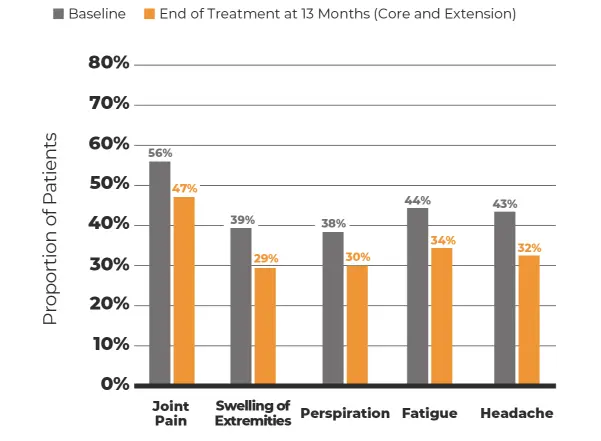
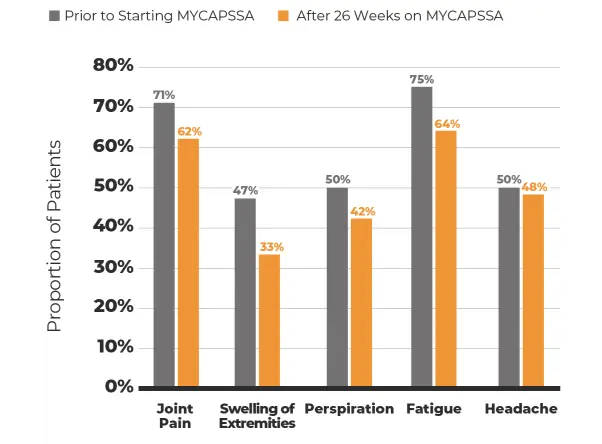
Treatment-related and treatment-emergent AEs occurring in ≥5% of patients in either treatment group during the randomized phase by system organ class and preferred term§§

Hypersensitivity to octreotide or any of the components of MYCAPSSA. Anaphylactoid reactions, including anaphylactic shock, have been reported in patients receiving octreotide.
MYCAPSSA can cause problems with the gallbladder. Monitor patients periodically. Discontinue if complications of cholelithiasis are suspected.
Blood sugar, thyroid levels, and vitamin B12 levels should be monitored and treated accordingly.
Bradycardia, arrhythmia, or conduction abnormalities may occur. Treatment with drugs that have bradycardia effects may need to be adjusted.
New onset of steatorrhea, stool discoloration, loose stools, abdominal bloating, and weight loss may occur with MYCAPSSA and other somatostatin analogs. If new occurrence or worsening of these symptoms are reported, evaluate for potential pancreatic exocrine insufficiency and manage accordingly.
The most common adverse reactions (incidence >10%) are nausea, diarrhea, headache, arthralgia, asthenia, hyperhidrosis, peripheral swelling, blood glucose increased, vomiting, abdominal discomfort, dyspepsia, sinusitis, and osteoarthritis.
The following drugs require monitoring and possible dose adjustment when used with MYCAPSSA: cyclosporine, insulin, antidiabetic drugs, calcium channel blockers, beta blockers, lisinopril, digoxin, bromocriptine, and drugs mainly metabolized by CYP3A4. Counsel women taking an oral contraceptive to use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA.
Patients taking proton pump inhibitors, H2-receptor antagonists, or antacids concomitantly with MYCAPSSA may require increased dosages of MYCAPSSA.
Advise premenopausal females of the potential for an unintended pregnancy.
Please report adverse events to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information, including Medication Guide.
MYCAPSSA (octreotide) delayed-release capsules, for oral use, is a somatostatin analog indicated for long-term maintenance treatment in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide.
Hypersensitivity to octreotide or any of the components of MYCAPSSA. Anaphylactoid reactions, including anaphylactic shock, have been reported in patients receiving octreotide.
MYCAPSSA can cause problems with the gallbladder. Monitor patients periodically. Discontinue if complications of cholelithiasis are suspected.
Blood sugar, thyroid levels, and vitamin B12 levels should be monitored and treated accordingly.
Bradycardia, arrhythmia, or conduction abnormalities may occur. Treatment with drugs that have bradycardia effects may need to be adjusted.
New onset of steatorrhea, stool discoloration, loose stools, abdominal bloating, and weight loss may occur with MYCAPSSA and other somatostatin analogs. If new occurrence or worsening of these symptoms are reported, evaluate for potential pancreatic exocrine insufficiency and manage accordingly.
The most common adverse reactions (incidence >10%) are nausea, diarrhea, headache, arthralgia, asthenia, hyperhidrosis, peripheral swelling, blood glucose increased, vomiting, abdominal discomfort, dyspepsia, sinusitis, and osteoarthritis.
The following drugs require monitoring and possible dose adjustment when used with MYCAPSSA: cyclosporine, insulin, antidiabetic drugs, calcium channel blockers, beta blockers, lisinopril, digoxin, bromocriptine, and drugs mainly metabolized by CYP3A4. Counsel women taking an oral contraceptive to use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA.
Patients taking proton pump inhibitors, H2-receptor antagonists, or antacids concomitantly with MYCAPSSA may require increased dosages of MYCAPSSA.
Advise premenopausal females of the potential for an unintended pregnancy.
Please report adverse events to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information, including Medication Guide.
MYCAPSSA (octreotide) delayed-release capsules, for oral use, is a somatostatin analog indicated for long-term maintenance treatment in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide.