

MYCAPSSA Support and FAQS


Jim's story of hope
Watch Jim as he talks about life with MYCAPSSA, and the support he's received along the way. Listen as he encourages others with acromegaly to advocate for themselves.

Becky's move to MYCAPSSA
Listen as Becky talks about transitioning to treatment with MYCAPSSA, and how she's made it fit into her everyday life.
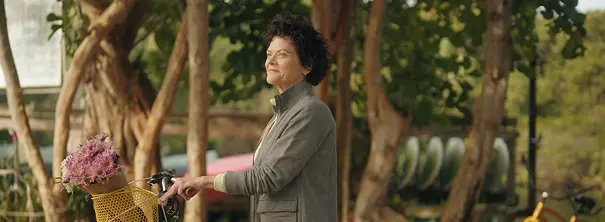
Bonnie's story of perseverance
Watch as Bonnie talks about her experience with acromegaly symptoms, and why she decided to switch to treatment with MYCAPSSA.
MYCAPSSA is the only octreotide that’s taken orally. MYCAPSSA is an oral prescription medicine approved for use in the long-term maintenance treatment of acromegaly in people who have responded to and tolerated treatment with octreotide or lanreotide.
If these treatments are effective and your body is tolerating it, you may be eligible to take MYCAPSSA instead of the injections.
MYCAPSSA is the oral version of octreotide, a medication you may already be familiar with.
Once swallowed with water, the coating on the capsule protects the medicine from being released too early or broken down before it gets to where it needs to be. The coating keeps the octreotide intact until it can make it to the right place in your body, your small intestine, to let the medication start to do its job.
MYCAPSSA is an oral form of octreotide, taken twice a day.
Somavert is a subcutaneous injection, self-administered once daily after a physician-supervised initial loading dose. Somatuline Depot is for deep subcutaneous injection only, initially administered every 4 weeks by a healthcare professional. Sandostatin LAR Depot and Signifor LAR are intramuscular injections, administered monthly by a healthcare professional.
Patients who respond to and tolerate octreotide or lanreotide may be eligible to take MYCAPSSA.
Once your doctor writes a prescription for MYCAPSSA, you will receive a call from Acaria Specialty Pharmacy to confirm your personal information, insurance information, and provide you with next steps. You may also enroll in the Chiesi Total CareSM patient support program, which provides additional services including individual support, insurance assistance, and worry-free refills. Learn more about Chiesi Total CareSM.
Once you start MYCAPSSA, your doctor may work with you to adjust your dose, depending on your IGF-I levels and your acromegaly signs and symptoms. The majority of patients in clinical trials received a 60-mg dose of MYCAPSSA or higher.
Make sure to have an open dialogue about how you feel, and let your doctor know how MYCAPSSA is working for you. This will help ensure that you are receiving the right amount of medicine to properly manage your acromegaly. Do not stop taking MYCAPSSA before speaking with your doctor or pharmacist.
†Somatuline Depot is a registered trademark of Ipsen Pharma S.A.S.
‡Sandostatin® is a registered trademark of Novartis Pharmaceuticals Corporation.
§Signifor® is a licensed trademark of Recordati Rare Diseases Inc.
||Please refer to the full Prescribing Information for each product for full Dosage and Administration information.



Important Safety Information
MYCAPSSA can cause problems with the gallbladder. Tell your healthcare provider if you have any of these symptoms: sudden pain in your upper right stomach (abdomen) or right shoulder or between your shoulder blades; yellowing of your skin or the whites of your eyes; fever with chills; or nausea.
MYCAPSSA may affect your blood sugar, thyroid hormone, or vitamin B12 levels. Tell your healthcare provider if you have any problems or conditions related to these. Your healthcare provider may monitor these levels during your treatment with MYCAPSSA.
Tell your healthcare provider if you have an irregular heartbeat.
MYCAPSSA may cause your body to have issues with absorbing dietary fats. Tell your healthcare provider if you have any new or worsening symptoms including fatty stools or stools with an oily appearance, changes in the color of your stools, loose stools, stomach (abdominal) bloating or weight loss.
Who should not use MYCAPSSA?
MYCAPSSA can cause a serious allergic reaction including anaphylactic shock. Stop taking MYCAPSSA right away and get emergency help if you have any of these symptoms: swelling of your tongue, throat, lips, eyes or face; trouble swallowing or breathing; severe itching of the skin with rash or raised bumps; feeling faint; chest pain; or rapid heartbeat.
Do not use MYCAPSSA if you are allergic to octreotide or any other ingredients in MYCAPSSA. If you need to know the ingredients, ask your healthcare provider or pharmacist.
If you have certain other medical conditions, you should use MYCAPSSA with caution. Tell your healthcare provider about all your medical conditions, especially the following: pregnancy or breastfeeding; liver disease; kidney disease; or difficulty in emptying bladder completely.
Women taking an oral contraceptive should use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA. Tell your healthcare provider about all the medicines you take. MYCAPSSA may affect the way other medicines work, and other medicines may affect how MYCAPSSA works.
What are the possible side effects of MYCAPSSA?
The most common side effects are headache, joint pain, nausea, weakness, diarrhea, and sweating a lot.
You are encouraged to report negative side effects to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Keep MYCAPSSA and all medicines out of the reach of children.
How should I take MYCAPSSA?
Do not take MYCAPSSA with food. MYCAPSSA should be taken with a glass of water on an empty stomach. Take MYCAPSSA at least 1 hour before a meal or at least 2 hours after a meal (for example, you could take your morning dose 1 hour before breakfast and your evening dose at bedtime).
Please see Full Prescribing Information, including Medication Guide, and talk to your doctor.
Indication
MYCAPSSA is an oral prescription medicine used in the long-term maintenance treatment of acromegaly in people for whom initial treatment with octreotide or lanreotide has been effective and tolerated.
If these treatments are effective and your body is tolerating it, you may be eligible to take MYCAPSSA instead of the injections. Ask your doctor if this oral treatment is appropriate for you.
Important Safety Information
MYCAPSSA can cause problems with the gallbladder. Tell your healthcare provider if you have any of these symptoms: sudden pain in your upper right stomach (abdomen) or right shoulder or between your shoulder blades; yellowing of your skin or the whites of your eyes; fever with chills; or nausea.
MYCAPSSA may affect your blood sugar, thyroid hormone, or vitamin B12 levels. Tell your healthcare provider if you have any problems or conditions related to these. Your healthcare provider may monitor these levels during your treatment with MYCAPSSA.
Tell your healthcare provider if you have an irregular heartbeat.
MYCAPSSA may cause your body to have issues with absorbing dietary fats. Tell your healthcare provider if you have any new or worsening symptoms including fatty stools or stools with an oily appearance, changes in the color of your stools, loose stools, stomach (abdominal) bloating or weight loss.
Who should not use MYCAPSSA?
MYCAPSSA can cause a serious allergic reaction including anaphylactic shock. Stop taking MYCAPSSA right away and get emergency help if you have any of these symptoms: swelling of your tongue, throat, lips, eyes or face; trouble swallowing or breathing; severe itching of the skin with rash or raised bumps; feeling faint; chest pain; or rapid heartbeat.
Do not use MYCAPSSA if you are allergic to octreotide or any other ingredients in MYCAPSSA. If you need to know the ingredients, ask your healthcare provider or pharmacist.
If you have certain other medical conditions, you should use MYCAPSSA with caution. Tell your healthcare provider about all your medical conditions, especially the following: pregnancy or breastfeeding; liver disease; kidney disease; or difficulty in emptying bladder completely.
Women taking an oral contraceptive should use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA. Tell your healthcare provider about all the medicines you take. MYCAPSSA may affect the way other medicines work, and other medicines may affect how MYCAPSSA works.
What are the possible side effects of MYCAPSSA?
The most common side effects are headache, joint pain, nausea, weakness, diarrhea, and sweating a lot.
You are encouraged to report negative side effects to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Keep MYCAPSSA and all medicines out of the reach of children.
How should I take MYCAPSSA?
Do not take MYCAPSSA with food. MYCAPSSA should be taken with a glass of water on an empty stomach. Take MYCAPSSA at least 1 hour before a meal or at least 2 hours after a meal (for example, you could take your morning dose 1 hour before breakfast and your evening dose at bedtime).
Please see Full Prescribing Information, including Medication Guide, and talk to your doctor.
Indication
MYCAPSSA is an oral prescription medicine used in the long-term maintenance treatment of acromegaly in people for whom initial treatment with octreotide or lanreotide has been effective and tolerated.
If these treatments are effective and your body is tolerating it, you may be eligible to take MYCAPSSA instead of the injections. Ask your doctor if this oral treatment is appropriate for you.





