






|
Study name
|
Study length |
Number of participants
|
What was studied |
|
OPTIMAL Study
|
9 months
|
56†
|
|
|
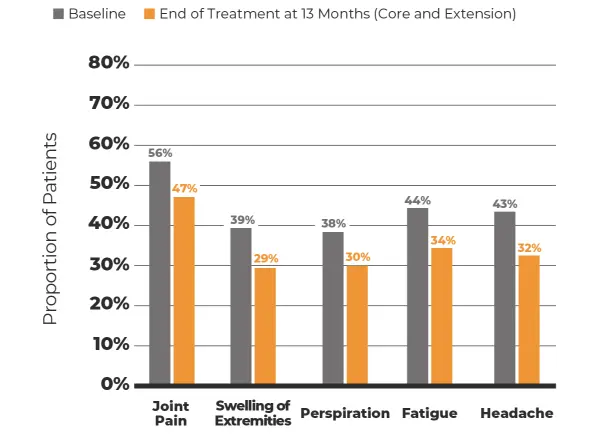
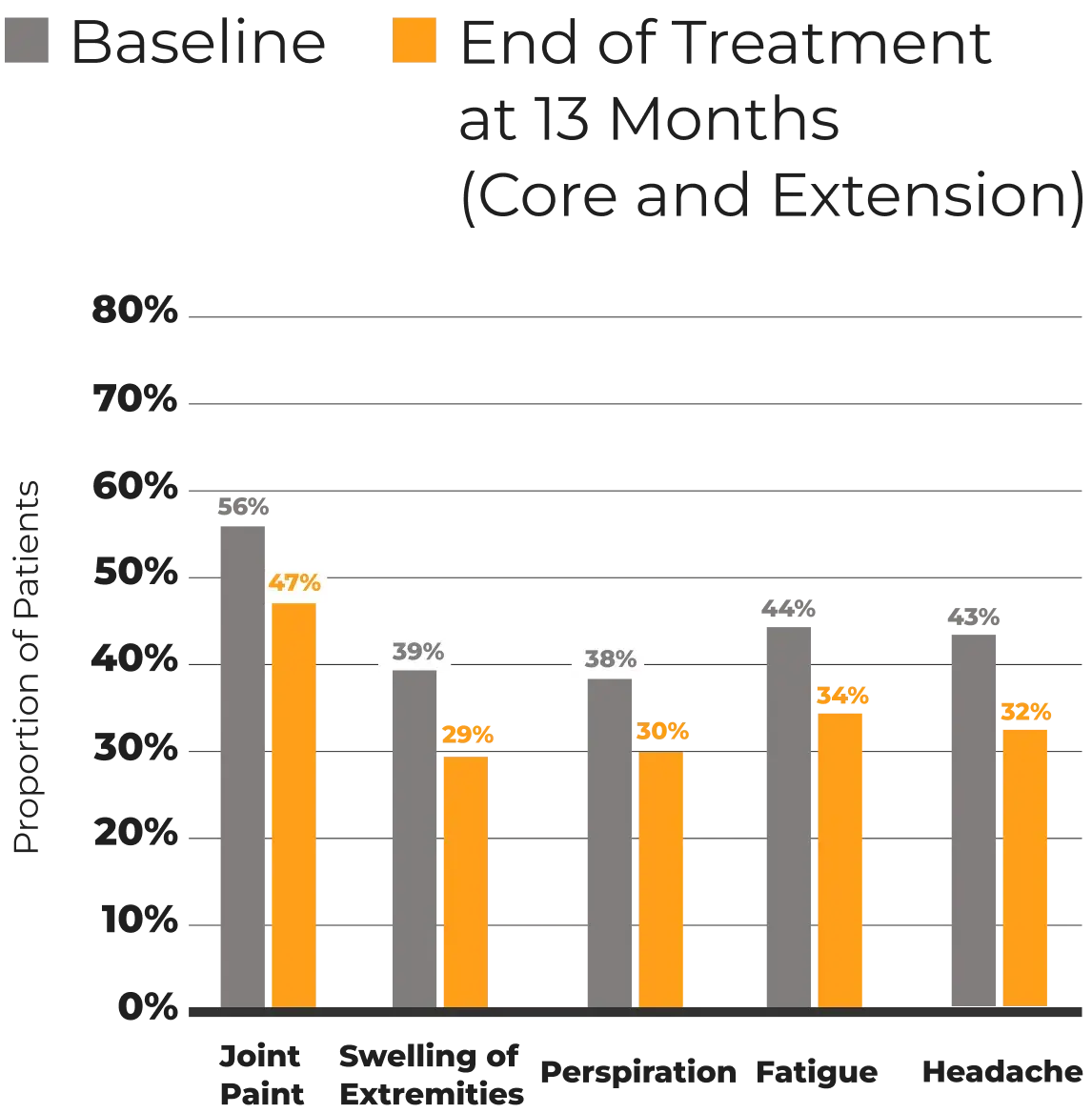
MPOWERED Study*
|
15 months
|
146‡
|
|
|
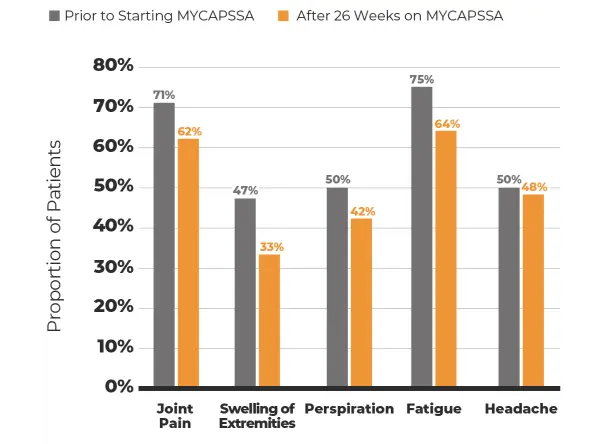
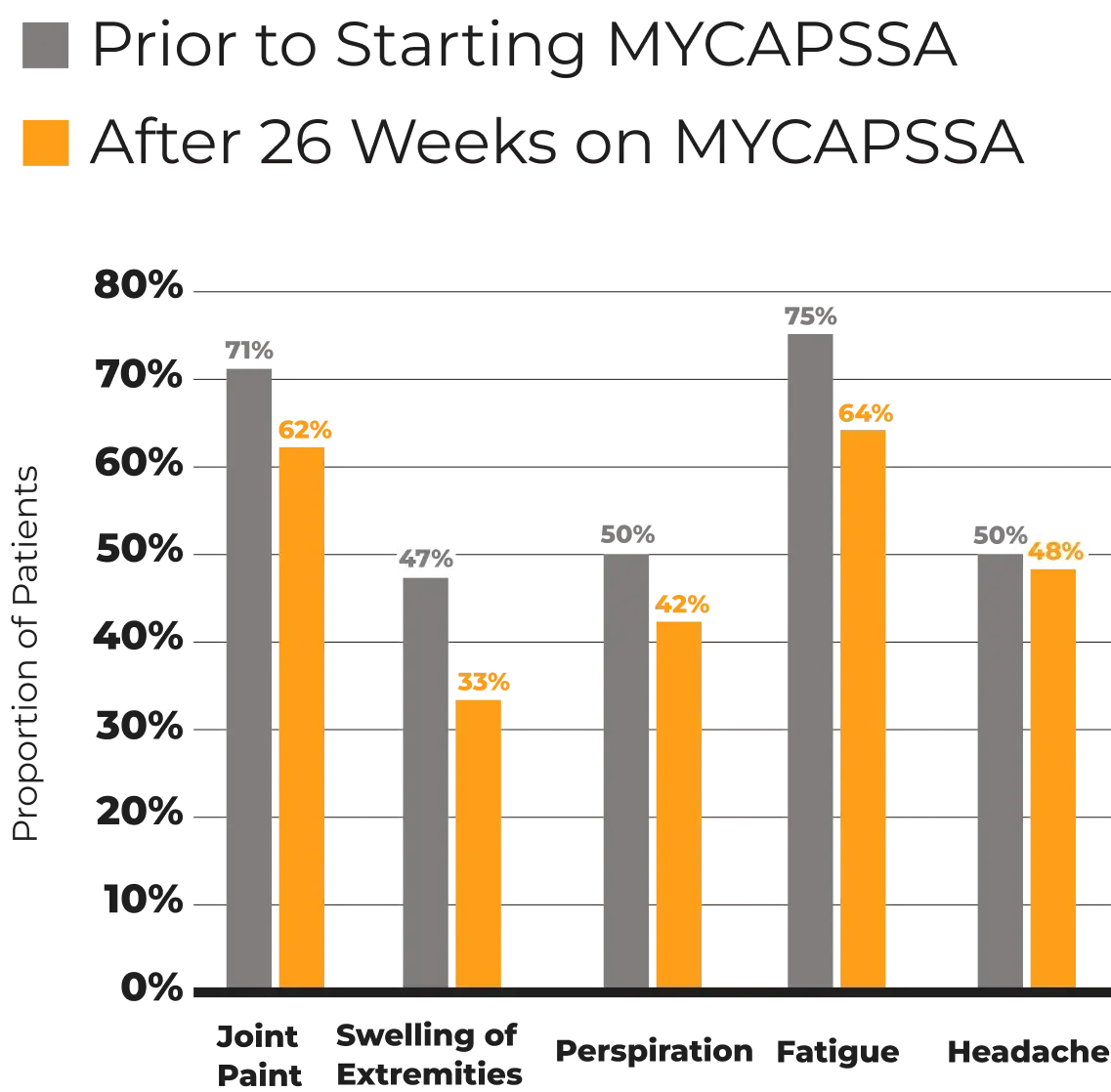
Open-Label Study
|
13 months
|
155
|
|
| Study name |
|
OPTIMAL Study
|
| Study length |
|
9 months
|
|
Number of participants
|
|
56†
|
| What was studied |
|
| Study name |
|
MPOWERED Study*
|
| Study length |
|
15 months
|
|
Number of participants
|
|
146‡
|
| What was studied |
|
| Study name |
|
Open-Label Study
|
| Study length |
|
13 months
|
|
Number of participants
|
|
155
|
| What was studied |
|




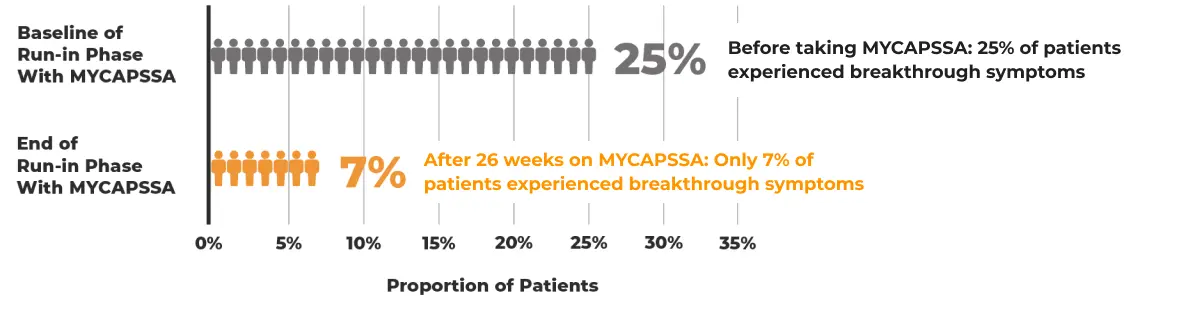




Stomach and gut issues were mostly mild to moderate and resolved on treatment.
Talk to your healthcare provider if you have any side effect that bothers you or that does not go away.
You may report side effects to the FDA at 1-800-FDA-1088.

MYCAPSSA can cause problems with the gallbladder. Tell your healthcare provider if you have any of these symptoms: sudden pain in your upper right stomach (abdomen) or right shoulder or between your shoulder blades; yellowing of your skin or the whites of your eyes; fever with chills; or nausea.
MYCAPSSA may affect your blood sugar, thyroid hormone, or vitamin B12 levels. Tell your healthcare provider if you have any problems or conditions related to these. Your healthcare provider may monitor these levels during your treatment with MYCAPSSA.
Tell your healthcare provider if you have an irregular heartbeat.
MYCAPSSA may cause your body to have issues with absorbing dietary fats. Tell your healthcare provider if you have any new or worsening symptoms including fatty stools or stools with an oily appearance, changes in the color of your stools, loose stools, stomach (abdominal) bloating or weight loss.
MYCAPSSA can cause a serious allergic reaction including anaphylactic shock. Stop taking MYCAPSSA right away and get emergency help if you have any of these symptoms: swelling of your tongue, throat, lips, eyes or face; trouble swallowing or breathing; severe itching of the skin with rash or raised bumps; feeling faint; chest pain; or rapid heartbeat.
Do not use MYCAPSSA if you are allergic to octreotide or any other ingredients in MYCAPSSA. If you need to know the ingredients, ask your healthcare provider or pharmacist.
If you have certain other medical conditions, you should use MYCAPSSA with caution. Tell your healthcare provider about all your medical conditions, especially the following: pregnancy or breastfeeding; liver disease; kidney disease; or difficulty in emptying bladder completely.
Women taking an oral contraceptive should use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA. Tell your healthcare provider about all the medicines you take. MYCAPSSA may affect the way other medicines work, and other medicines may affect how MYCAPSSA works.
The most common side effects are headache, joint pain, nausea, weakness, diarrhea, and sweating a lot.
You are encouraged to report negative side effects to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Keep MYCAPSSA and all medicines out of the reach of children.
Do not take MYCAPSSA with food. MYCAPSSA should be taken with a glass of water on an empty stomach. Take MYCAPSSA at least 1 hour before a meal or at least 2 hours after a meal (for example, you could take your morning dose 1 hour before breakfast and your evening dose at bedtime).
Please see Full Prescribing Information, including Medication Guide, and talk to your doctor.
MYCAPSSA is an oral prescription medicine used in the long-term maintenance treatment of acromegaly in people for whom initial treatment with octreotide or lanreotide has been effective and tolerated.
If these treatments are effective and your body is tolerating it, you may be eligible to take MYCAPSSA instead of the injections. Ask your doctor if this oral treatment is appropriate for you.
MYCAPSSA can cause problems with the gallbladder. Tell your healthcare provider if you have any of these symptoms: sudden pain in your upper right stomach (abdomen) or right shoulder or between your shoulder blades; yellowing of your skin or the whites of your eyes; fever with chills; or nausea.
MYCAPSSA may affect your blood sugar, thyroid hormone, or vitamin B12 levels. Tell your healthcare provider if you have any problems or conditions related to these. Your healthcare provider may monitor these levels during your treatment with MYCAPSSA.
Tell your healthcare provider if you have an irregular heartbeat.
MYCAPSSA may cause your body to have issues with absorbing dietary fats. Tell your healthcare provider if you have any new or worsening symptoms including fatty stools or stools with an oily appearance, changes in the color of your stools, loose stools, stomach (abdominal) bloating or weight loss.
MYCAPSSA can cause a serious allergic reaction including anaphylactic shock. Stop taking MYCAPSSA right away and get emergency help if you have any of these symptoms: swelling of your tongue, throat, lips, eyes or face; trouble swallowing or breathing; severe itching of the skin with rash or raised bumps; feeling faint; chest pain; or rapid heartbeat.
Do not use MYCAPSSA if you are allergic to octreotide or any other ingredients in MYCAPSSA. If you need to know the ingredients, ask your healthcare provider or pharmacist.
If you have certain other medical conditions, you should use MYCAPSSA with caution. Tell your healthcare provider about all your medical conditions, especially the following: pregnancy or breastfeeding; liver disease; kidney disease; or difficulty in emptying bladder completely.
Women taking an oral contraceptive should use an alternative non-hormonal method of contraception or a back-up method when taking MYCAPSSA. Tell your healthcare provider about all the medicines you take. MYCAPSSA may affect the way other medicines work, and other medicines may affect how MYCAPSSA works.
The most common side effects are headache, joint pain, nausea, weakness, diarrhea, and sweating a lot.
You are encouraged to report negative side effects to Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Keep MYCAPSSA and all medicines out of the reach of children.
Do not take MYCAPSSA with food. MYCAPSSA should be taken with a glass of water on an empty stomach. Take MYCAPSSA at least 1 hour before a meal or at least 2 hours after a meal (for example, you could take your morning dose 1 hour before breakfast and your evening dose at bedtime).
Please see Full Prescribing Information, including Medication Guide, and talk to your doctor.
MYCAPSSA is an oral prescription medicine used in the long-term maintenance treatment of acromegaly in people for whom initial treatment with octreotide or lanreotide has been effective and tolerated.
If these treatments are effective and your body is tolerating it, you may be eligible to take MYCAPSSA instead of the injections. Ask your doctor if this oral treatment is appropriate for you.